Ophthalmology
Faricimab (Vabysmo) for Retinal Vein Occlusion (RVO)
Patient Information leaflet
Introduction
Your eye doctor has already given you a patient information leaflet called ‘A Guide to Retinal Vein Occlusion’ describing retinal vein occlusion (RVO), the treatment and where to find further information.
This leaflet contains detailed information on a treatment with the medical name of ‘Faricimab’, also known as ‘Vabysmo’. The leaflet includes information on the procedure, the risks and the benefits.
If you suffer from glaucoma / ocular hypertension, or have had an ocular hypertensive response to a previous Ozurdex implant and have been enlisted for intravitreal Faricimab (Vabysmo) RVO injections, please continue to take your prescribed glaucoma eye drops whilst having these injections.
What is Faricimab (Vabysmo)?
If your doctor is suggesting Faricimab (Vabysmo) treatment, it means your eye contains extra amounts of the proteins, vascular endothelial growth factor (VEGF) and angiopoietin (ANG 2). They are the cause of leaky, abnormal blood vessels. The excess fluid that comes from these blood vessels can build up and lead to reduction in your vision.
Faricimab (Vabysmo) is designed to block VEGF and ANG 2. By blocking these, it may prevent damaged blood vessels from leaking fluid into the macula. It is given by a course of injections into the eye. Long term, the number of injections will depend on how the RVO responds to the treatment.
Your doctor has found that you have retinal vein occlusion (RVO) and need to be started on eye injections to treat it.
These injections are currently one of the most effective treatments for RVO. They work by penetrating the nerve layer at the back of the eye (the retina). The macula is the most important part of the retina and is responsible for your central vision. Over time, the injections close the leaking blood vessels affecting the macula, which should reduce the swelling in the macula and hopefully improve your vision.
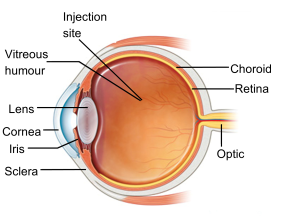
Figure 1 shows the side image of an eye (image courtesy of NHS Choices).
Will my vision improve with the injection?
BALATON and COMINO: Phase III Trials:
- This pivotal trial showed that Faricimab (Vabysmo) was equally effective (non-inferior) when tested against Aflibercept (Eylea or Yesafili).
- The vision gained (assessed as three lines improvement on the vision chart) and vision lost (three lines less on the vision chart) from baseline, was similar in both groups of patients tested with the two drugs.
- Faricimab (Vabysmo) had a better drying effect compared to Aflibercept (Eylea or Yesafili) in the two groups (12 per cent more in BALATON – Branch Vein Occlusion and 14 per cent more in COMINO – Central Retinal Vein Occlusion).
- Faricimab (Vabysmo) and Aflibercept (Eylea or Yesafili) were well tolerated in terms of side effects. No cases of Endophthalmitis (serious intraocular inflammation) or retinal vasculitis were reported in Faricimab (Vabysmo) treated patients
- Risk of central artery occlusion / thrombosis was 0.6 per cent in Aflibercept (Eylea or Yesafili) injections compared to 0.8 per cent in Faricimab (Vabysmo).
- Risk of cardiovascular events (Heart attack and Stroke) was 1.4 per cent in Aflibercept and 1.1 per cent in Faricimab injection.
- Similar percentage of patients dropped out (discontinued) treatment within six months of the trial, 0.4 per cent in Branch Vein Occlusion and 0.8 per cent in Central Retinal Vein Occlusion for various reasons.
Faricimab (Vabysmo) injections are given as one every four weeks for the first six months (this is called a loading dose), followed by a personalised treatment interval (PTI) of an injection every two, three or four months in year one. In the subsequent years, the injection is given on physician (eye doctor) discretion, just like Aflibercept (Eylea or Yesafili). The number and frequency of the injections and the overall duration of treatment depend on the diagnosis and severity of the condition. Many patients have to have the injections for up to three years or longer. Your doctor will choose and discuss the treatment plan (regimen) best suited to your eye.
How long am I consenting for treatment?
You will be given an indefinite course of treatment, unless you withdraw consent or lose capacity.
What happens during the treatment?
You should not feel any pain during the eye injections, since your eye is numbed with anaesthetic drops prior to the injections. You may feel some pressure on your eye. You will not need to stay in hospital.
After the treatment
You can take a couple of paracetamol tablets (500mg) in the morning of the injection or afterwards (if not allergic) if necessary.
Please continue to take any other eye drops that you already use (e.g. for glaucoma or dry eye). After the injection, the eye will be covered by an eye shield to prevent corneal scratch / abrasion. Please keep the shield on the eye until the next morning.
What are the benefits?
The benefits of the treatment are:
- The injections should reduce inflammation and swelling in the macula of your eye.
- It can also help improve vision and prevent further damage.
What are the risks of having the injections?
You need to know about the side effects. Most of the side effects are mild to moderate and will generally disappear within a week after each injection. Contact your doctor if any of the following side effects become severe. Most treatments have some risks. The risks of Faricimab (Vabysmo) are similar to the other anti VEGF injections and are as follows:
Very common (may affect more than one in ten people):
- Cloudy lens in the eye (cataract)
Common (may affect up to one in ten people):
- Tearing of the retina (the layer at the back of the eye that detects light) or one of its layers
- Increase in pressure inside the eye (intraocular pressure increase)
- Bleeding from small blood vessels in the outer layer of the eye (conjunctival haemorrhage)
- Moving spots or dark shapes in your vision (vitreous floaters)
- Eye pain
- Increase tear production (lacrimation increased)
Uncommon (may affect up to one in 100 people):
- Serious inflammation inside the eye.
- Inflammation of the gel-like substance inside the eye (vitritis)
- Inflammation in the iris and its adjacent tissue in the eye (iritis, iridocyclitis, uveitis)
- Bleeding in the eye (vitreous haemorrhage)
- Scratched cornea, damage to the clear layer of the eyeball that covers the iris (corneal abrasion)
- Eye irritation
- Eye discomfort
- Itching (eye pruritus)
- Red eye (ocular/conjunctival hyperaemia)
- A feeling of having something in your eye
- Decreased sharpness of vision (visual acuity reduced)
- Two to three people in 100 can have, a stroke, mini stroke, heart attack or hear failure. There is no proven link between this injection and these possible side effect.
Rare (may affect up to one in 1,000 people):
- Detachment of the retina
- Temporary decreased sharpness of vision (visual acuity reduced transiently)
- Less than 0.5 per cent of patients may have an eye infection (Endophthalmitis), leading to complete loss of vision (blindness).
Reporting of side effects
If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. You may also report side effects directly (see details below). By reporting side effects, you can help provide more information for the safety of this medicine.
United Kingdom – Yellow card scheme
Website: www.mhra.gov.uk/yellowcard
What are the alternatives?
Currently, other licensed anti-VEGF treatments for RVO apart from Faricimab (Vabysmo) are Aflibercept (Eylea or Yesafili) and Ranibizumab (Ongavia) intravitreal injections. Steroid implants Dexamethasone (Ozurdex) is also licensed for RVO.
Is there any reason why I cannot have the injections?
- The injections cannot be given to people who have had a stroke, mini-stroke (TIA) or heart failure in the past three months.
- They will not be used in the presence of infection / inflammation in or around the surrounding tissues of the eye.
- The injections are unsuitable in pregnancy and breast-feeding women.
- Additional support may be needed for patients who may find local anaesthetics difficult to tolerate due to dementia/cognitive impairment. In this case, alternative solutions will be discussed with the patient and those who support them.
‘One Stop Service’
The Trust is introducing a ‘One Stop Service’ for some intravitreal injections. A ‘One Stop Service’ is where an injection may be offered on the same day you attend the eye clinic. This may result in you having an extended waiting time in clinic, but it will mean you do not have to return on a separate occasion for your eye injection. A doctor or nurse will discuss this with you in the clinic.
Advice after eye injections
What should I expect after the injection?
Your eye may feel painful for 24 to 48 hours. If necessary, you can take painkillers such as paracetamol or ibuprofen if you can take them (always read the label; do not exceed the recommended dose). If the eye becomes significantly red and painful with reduced vision, contact the Urgent Referral team immediately on 01384 456111 ext. 3633.
It is best to avoid products containing aspirin. However, if you take regular soluble aspirin (75mg), you can continue to take it as advised by your GP.
If you have bruising on or around the eye, this should fade gradually over the next couple of weeks.
At times, a tiny air bubble can be introduced into the eye during the injection. This appears as a round, dark floater in the centre of your vision the day after the injection. Do not be alarmed, as this will get smaller and should disappear within 48 hours.
Rarely, the surface of the eye can get scratched during the injection process. This can cause sharp, sudden pain three to six hours after the injection. If this happens, it is easy to treat, so please get in touch with the Urgent Referral team at Russells Hall Hospital Eye Clinic on 01384 456111 ext. 3633 (9am to 4.30pm, Monday to Friday).
What do I need to do?
If you have an eye pad to prevent the cornea from being scratched or damaged, you can gently remove this the next morning. The eye pad may be slightly bloodstained, but this is nothing to worry about.
You can clean your eye the morning after your injection with cool, boiled water and a small piece of cotton wool or lint. Close your eye first, and then gently wipe from the inner corner of the eye to the outer corner of the eye, using a fresh piece of cotton wool or lint each time and for each eye.
If you were prescribed antibiotic drops to use at home, continue to use them for five days. If you have been prescribed glaucoma eye drops, you should use them on the morning of the injection, but not after the injection for the rest of that day. The next day you should start your glaucoma eye drops again using a new bottle.
What if I have any problems or questions after reading this leaflet?
Please contact the Urgent Referral Clinic team at Russells Hall Hospital Eye Clinic on 01384 456111 ext. 3633 (9am to 4.30pm, Monday to Friday).
Eye emergency, out of hours
In case of an eye emergency after the closing hours of the Eye Clinic at
Russells Hall Hospital (including weekends and bank holidays), please contact:
Birmingham and Midland Eye Centre on 0121 507 4440
The doctor on call is usually based at the Eye Centre, City Hospital, Dudley Road, Birmingham. They may need to call you back, and if necessary, they will arrange for you to visit them.
Where can I find out more?
You can find out more from the following web-link:
NHS Choices
http://www.nhs.uk/Conditions/Macular-degeneration/Pages/introduction.aspx
Note: the information in this booklet is provided for information only. The information found is not a substitute for professional medical advice or care by a qualified doctor or other health care professional. Always check with your doctor if you have any concerns about your condition or treatment. This is only indicative and general information for the procedure. Individual experiences may vary and all the points may not apply to all patients at all times. Please discuss your individual circumstances with your eye doctor.
Author: Mr S Shafquat FRCS FRCOphth Consultant Ophthalmologist, Retina Lead
Reference
www.nice.org.uk/guidance/tag1004 (published 11th September 2024).
BALATON and COMINO: Phase III Randomised Clinical Trials of Faricimab for Retinal Vein Occlusion.
If you have any questions, or if there is anything you do not understand, please contact the Russells Hall Hospital switchboard number on 01384 456111 and ask for the relevant department who issued this leaflet.
If you have any feedback on this patient information leaflet please email dgft.patient.information@nhs.net
This leaflet can be made available in large print, audio version and in other languages, please call 0800 073 0510.
Faricimab (Vabysmo)/RVO/SS/ST/DP 12 2025/v2 – review 09 2028 DGH/PIL/02174
